The Sunday Mail

 People living with hiv are at risk of increased medical complications following the unregulated entry into the country of the latest generation of anti-retroviral drugs (arvs), which have become popular as they can be consumed more conveniently.
People living with hiv are at risk of increased medical complications following the unregulated entry into the country of the latest generation of anti-retroviral drugs (arvs), which have become popular as they can be consumed more conveniently.
Investigations last week showed that the new drugs are in short supply at formal dispensaries and are now being brought into the country illegally.
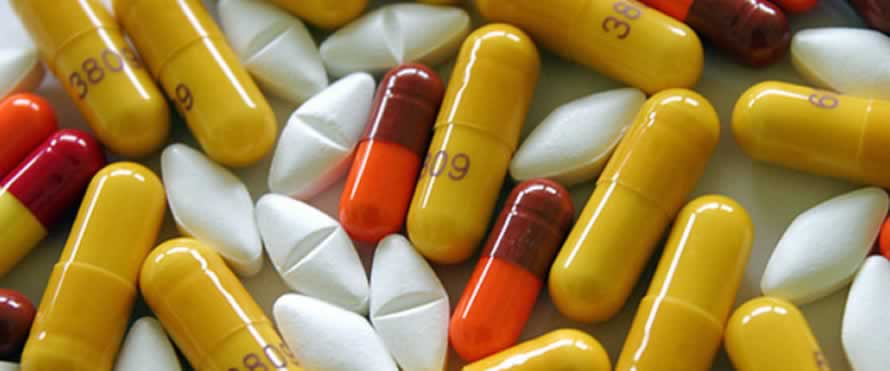
Those living with hiv prefer the drugs which are packaged as single tablets consisting of three dosages.
The previous regimen required one to take at least six tablets per day. However, the new generation medication means taking one tablet per day.
Zimbabwe is still offering a free combination of at least four tablets a day, a situation that has seen people living with hiv resorting to unregulated alternative sources.
Only expecting mothers and tuberculosis patients are accessing the single tablet under a Government-funded exercise.
Health experts say expecting mother’s living with hiv are being provided with a single tablet which contains either Tenofovir or Zidovine combined with Lamivudine and Nevirapine.
TB patients living with hiv get a single tablet of Tenorlum E, which is a combination of Tenofovir, Efavirenz and Lamivudine.
The Sunday Mail has gathered that the latest generation of arvs is smuggled into the country from Zambia and South Africa.
Investigations show the smuggled drugs are sold at between $15 to $18 which is half of the pharmaceutical price which ranges between $30 and $40 for a month’s supply.
National Aids Council (nac) monitoring and evaluation manager Mr Amon Mpofu could neither confirm nor deny the illegal influx of the drugs.
“We can neither deny nor agree that the drugs are being smuggled because we have not yet received any formal report,” Mr Mpofu said.
“If that is the case, then people accessing these drugs outside pharmacies and Government health institutions are at high risk of developing side effects or defaulting treatment resulting into the development of opportunistic infections.
“Our fears are that no one knows if these drugs are standard or safe for human consumption.”
Mr Mpofu said the country could not provide the single tablets to all people living with hiv because of inadequate funds. He said nac was yet to receive additional funding from the Global Fund to procure the new drugs.
Mr Mpofu said: “Although we are procuring (latest combinations of arvs) for a small group, the process (to receive the drugs) takes longer and costly compared to the previous combinations.”
nac estimates about 1,4 million people in Zimbabwe are living with hiv and are on anti-retroviral treatment (art).
Of this figure, about 156 718 are children.
The first anti-retroviral drug administered to people living with HIV in Zimbabwe was Zidovudine (azt or zdv), which was introduced in 1996 as a single drug.
Several drugs have been introduced since.Anti-retroviral drugs were first dispensed for free in the country in 2004 under a Government initiative.
According to the Ministry of Health and Child Care hiv and aids and TB Unit, the early first line drug combination was Stavudine (d4T), Lamuvidine (3TC) and Nevirapine (nvp).
During that time, people living with HIV and were only initiated on Art if their CD4 count was less than 200, as per World Health Organisation (who) guidelines.
However, the count was increased to 350 in 2010.
“The early first line drug combination was later reviewed in 2010 when Stavudine was replaced with Tenofovir because most people were experiencing side effects from the drug,” the director of the hiv, aids and tb Unit, Dr Owen Mugurungi, said.
Who recommended Tenofovir and the country adapted to a combination of Tenofovir plus Lamuvidine and Nevirapine 3.
Dr Mugurungi said the latest who guidelines launched last year, recommend the use of one tablet per day (a combination of Tenofovir, Lamuvudine and Efavirenz).
These guidelines were launched together with new hiv guidelines that suggest people living with hiv should be initiated on Art if their CD4 count is less than 500.
Information from Who shows that infected pregnant mothers used to take Zidovudine plus SD Nevirapine (azt) at 28 weeks of pregnancy in 2004.
In 2006, these mothers moved to an additional SD Nevirapine (nvp) plus Lamuvidine and continue with azt.
Option A was introduced to these mothers in 2010 where they were given azt and infant nvp.



